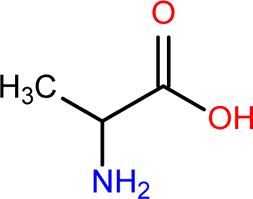
Pyruvate family: Alanine (A)
http://www.bmrb.wisc.edu/metabolomics/mol_summary/?molName=D_alanine
pKa of R group: effectively NA
MW: ~90 g/mol
Physiological roles
Converted to beta-alanine and joined with pentoate to give pantothenoic acid. Pantothenoic acid is the core component of Coenzyme-A, one of the most frequently used coenzymes that uses its thioester bond-forming capabilities to drive reactions for numbers of what otherwise would be unfavorable reactions. Here is the breakdown of coenzyme A:
http://lipidlibrary.aocs.org/lipids/coa/index.htm
To created the strucuture of CoA alanine units need to be converted to beta-alanine, which is a disjointed form of the amino acid, with the amino group on the beta carbon as opposed to the 'classical' alpha carbon.
http://chembase.com/image_structures-00000001_00025000-13351.png
Bacterial synthesis of beta-alanine uses L-aspartate as shown here:
http://lipidlibrary.aocs.org/lipids/coa/index.htm
To created the strucuture of CoA alanine units need to be converted to beta-alanine, which is a disjointed form of the amino acid, with the amino group on the beta carbon as opposed to the 'classical' alpha carbon.
http://chembase.com/image_structures-00000001_00025000-13351.png
Bacterial synthesis of beta-alanine uses L-aspartate as shown here:
From there, beta-alanine can be incorporated into this very important compound (there are different enzymes that catalyze this reaction in eukaryotes).
There are beta-alanine analogs that are thought to be good potential chemotherapeutics. Details to come. . .
There are beta-alanine analogs that are thought to be good potential chemotherapeutics. Details to come. . .
Alanine is ‘best known’ in the microbial world for its dipeptide bond formation of D-ala-D-ala at the end of the pentapeptide extension from N-acetylmuramic acid (NAM) residues of peptidoglycan polymers. This bond is used for the tetrapeptide linkage of opposite facing NAM residues. L-alanine is synthesized and alanine racemase converts it to its stereoisomer, D-alanine. Two D-alanines are then joined by their respective ligase.
The R groups in this case are -CH3 (methyl) groups, and though chirality is not shown, in this case, the configurations are both D. (Need a refresher on chirality? Here is some help with the CORN rule: http://dwb4.unl.edu/Chem/CHEM869K/CHEM869KLinks/www.ccp14.ac.uk/ccp/web-mirrors/llnlrupp/Xray/tutorial/protein_structure.htm )
The peptidoglycan transpeptidase enzyme (also called Penicillin Binding Protein (PBP) because it will covalently bind beta-lactam rings characteristic of penicillins) will covalently link two bridges (the bridges are present in gram positive bacteria, but between gram-negatives there is no bridge between the tetrapeptide extenstions from NAM units). Here is a good summary of the polymerization of peptidoglycan and cross-linking:
Also, interesting is the fact that tmRNA used in trans-translation is only charged with alanine. Details about how alanine-charged tmRNA will disassemble stalled ribosomes:
http://129.123.92.202/biol5190/PDFs/annurev_tmRNA.pdf
http://129.123.92.202/biol5190/PDFs/annurev_tmRNA.pdf
Synthesis
Most often alanine is synthesized by the reductive amination of pyruvate, but there are other enzymes for which there is support of their function in alanine synthesis. Just in Escherichia coli, there are likely two other pathways that are used to add to the alanine pool. The most supported enzyme is a glutamate-pyruvate aminotransferase, that uses the common alpha amino donor, glutamate to add an amino group to pyruvate (this is the same as what occurs in eukaryotes)
Directly from an another amino acid, another pathway does exist in E. coli,
Bacillus subtilis, and Homo sapiens. This pathway does not aminate a three carbon compound (pyruvate) but will desulfonate a cysteine. To visualize this look at the structure of cysteine.
http://ecocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY0-1021&detail-level=2
Directly from an another amino acid, another pathway does exist in E. coli,
Bacillus subtilis, and Homo sapiens. This pathway does not aminate a three carbon compound (pyruvate) but will desulfonate a cysteine. To visualize this look at the structure of cysteine.
http://ecocyc.org/META/NEW-IMAGE?type=PATHWAY&object=PWY0-1021&detail-level=2





No comments:
Post a Comment